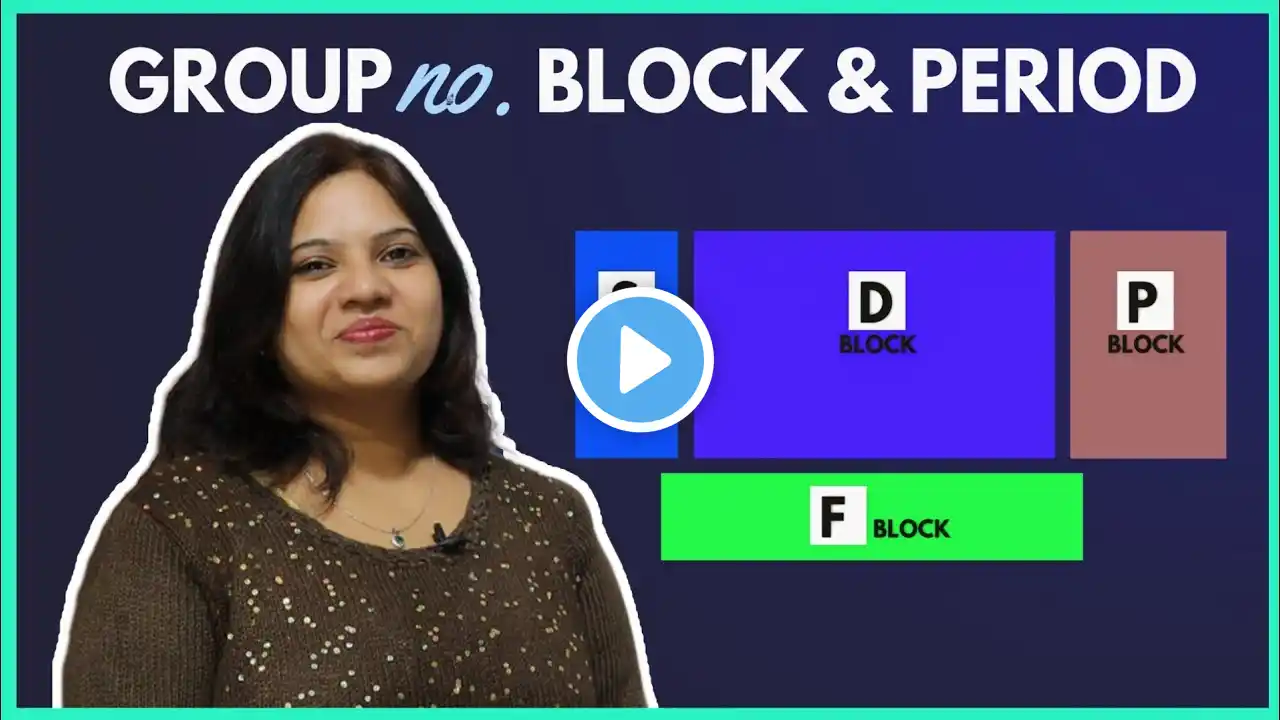
How to find Group, Period and Block of an element?
This lecture is about how to find group, period, block and subgroup of any element. I will teach you the super easy trick through which you can find the group, period and subgroup of s-block elements, p-block elements and d-block elements. For example, consider Sodium and its atomic number is 11. Firstly, we will configure the electrons of sodium. The last electron of sodium is in the s orbital. So, sodium is s-block element. The highest principal quantum number of sodium is 3. So, sodium is present in the third period. There are only one electron in the last s-orbital of sodium. So, sodium is in the first group. The last orbital of sodium is "s". So, its subgroup is 1A. Using this trick, we can calculate the group number, period numbers, subgroup number and block number of any element in the periodic table. In this animated lecture, you will learn about the groups, periods and blocks of the following elements with examples. 1) Sodium 2) Calcium 3) Magnesium 4) Nitrogen 5) Iron 6) Zinc 7) Nickel 8) Bromine To learn more about the trick of finding group, period and block of any element with examples, watch this lecture till the end. SPDF Electronic Configuration: • SPDF Electronic Configuration Trick |... #GroupNumberAndPeriodNumber #TrickToFindGroupandPeriodNumber #Chemistry Subscribe my channel at: / @najamacademy Youtube link: / @najamacademy Facebook link: / najamacademy