Changing Kp | A-Level Chemistry | AQA, OCR, Edexcel
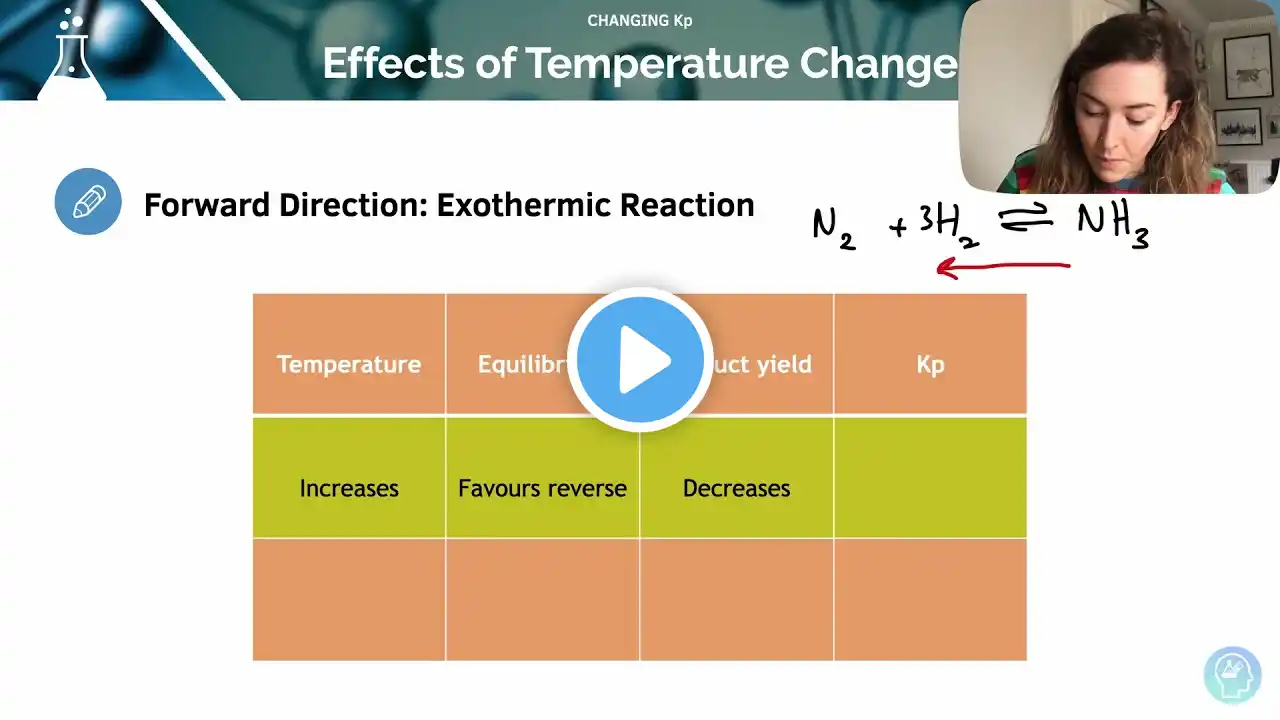
How Temperature Affects the Value Kp Changing the temperature of a reaction also changes the value of Kp because changing the temperature changes the amount of product formed. This in turn changes the number of moles on each side of the equation, therefore the mole fractions and the partial pressure of each reactant is different. This changes the value of Kp that is calculated. Forward direction: exothermic reaction • Increase the temperature - Equilibrium shifts in the reverse direction, to favor the endothermic reaction, and so reduce the temperature. The yield of products decreases. The partial pressures of the products will decrease, therefore Kp will decrease. • Decrease the temperature - Equilibrium shifts in the forward direction, to favor the exothermic reaction, and increase the temperature. More products are formed. The partial pressure of the products will increase, therefore Kp will increase. Forward direction: endothermic reaction • Increase the temperature - Equilibrium shifts in the forward direction, to favor the endothermic reaction and reduce the temperature. More products are formed. The partial pressure of the products will increase, therefore Kp will increase. • Decrease the temperature - Equilibrium shifts in the reverse direction, to favor the exothermic reaction and increase temperature. The yield of products decreases. The partial pressures of the products will decrease, therefore Kp will decrease. Summary Type of reaction Temperature change Effect on Kp Exothermic Increases Decreases Exothermic Decreases Increases Endothermic Increases Increases Endothermic Decreases Decreases Effect of Pressure and Catalysts on Kp Effect of adding a Catalyst Similar to Kc, adding a catalyst does not affect the value of Kp. A catalyst simply allows equilibrium to be reached sooner. A catalyst will affect the rate of reaction in both directions by the same amount. Effect of changing the Pressure Pressure does not affect the value of Kp, just as concentration does not affect the value of Kc. An increase in pressure causes equilibrium to shift in favor of the direction with the fewer moles so that the pressure decreases. The partial pressure ratio of reactant to products stays the same so Kp does not change. 🔓 Unlock Full Course: https://courses.medicmind.co.uk/p/ale... 🔖 Past Papers: https://www.studymind.co.uk/resources/ 👩🏻🏫 1-1 Tutoring: https://www.studymind.co.uk/subject/a... #ALevelChemistry #Chemistry #A-LevelChemistry #StudyMind

