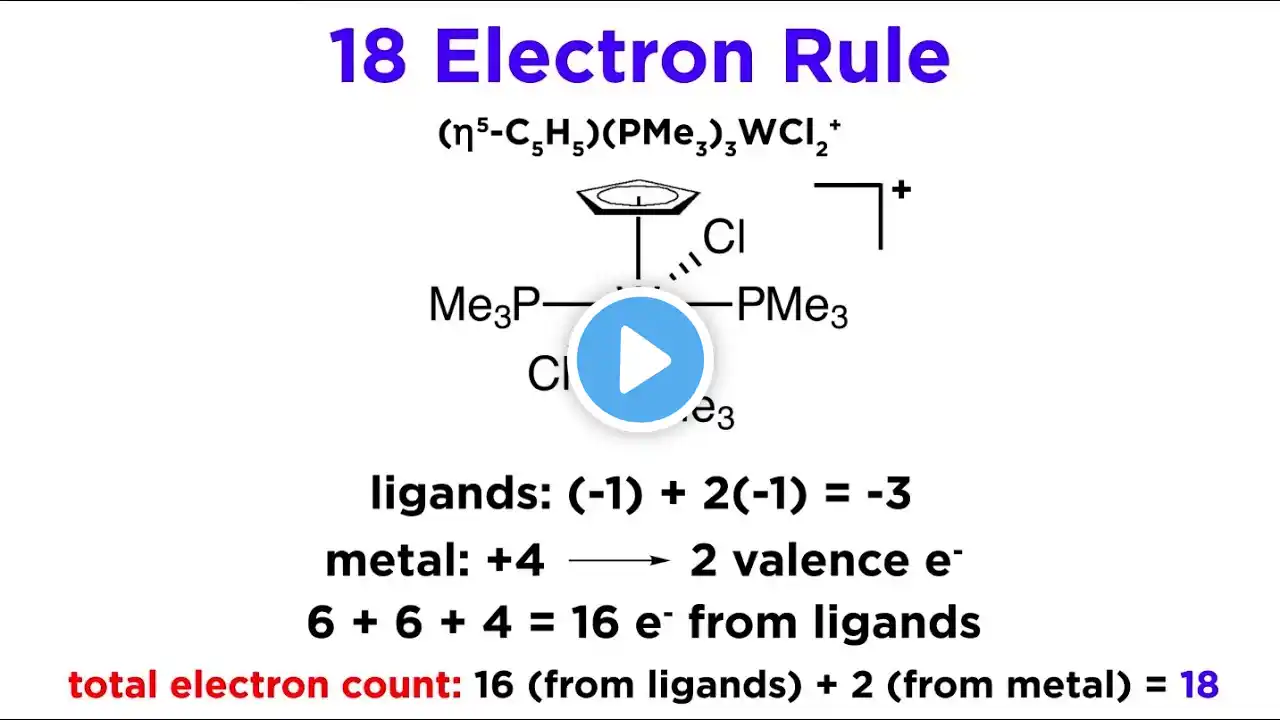
The 18 Electron Rule for Transition Metal Complexes
Ok, so we understand how ligands bond to metals to form transition metal complexes, but how many ligands will fit? Well, remember the octet rule from general chemistry? There is a similar concept that we can apply here, which is called the 18 electron rule. 18 is bigger than 8 because transition metals have access to d orbitals. Let's see how this rule works now! Watch the whole Inorganic/Organometallic Chemistry playlist: http://bit.ly/ProfDaveInorganic General Chemistry Tutorials: http://bit.ly/ProfDaveGenChem Organic Chemistry Tutorials: http://bit.ly/ProfDaveOrgChem Biochemistry Tutorials: http://bit.ly/ProfDaveBiochem Biology Tutorials: http://bit.ly/ProfDaveBio Classical Physics Tutorials: http://bit.ly/ProfDavePhysics1 Modern Physics Tutorials: http://bit.ly/ProfDavePhysics2 Mathematics Tutorials: http://bit.ly/ProfDaveMath EMAIL► [email protected] PATREON► / professordaveexplains Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience! Amazon: https://amzn.to/2HtNpVH Bookshop: https://bit.ly/39cKADM Barnes and Noble: https://bit.ly/3pUjmrn Book Depository: http://bit.ly/3aOVDlT