CHEM#23 ~ CXC/CSEC CHEMISTRY JUNE 2013 Paper 1 ~ Revision#2
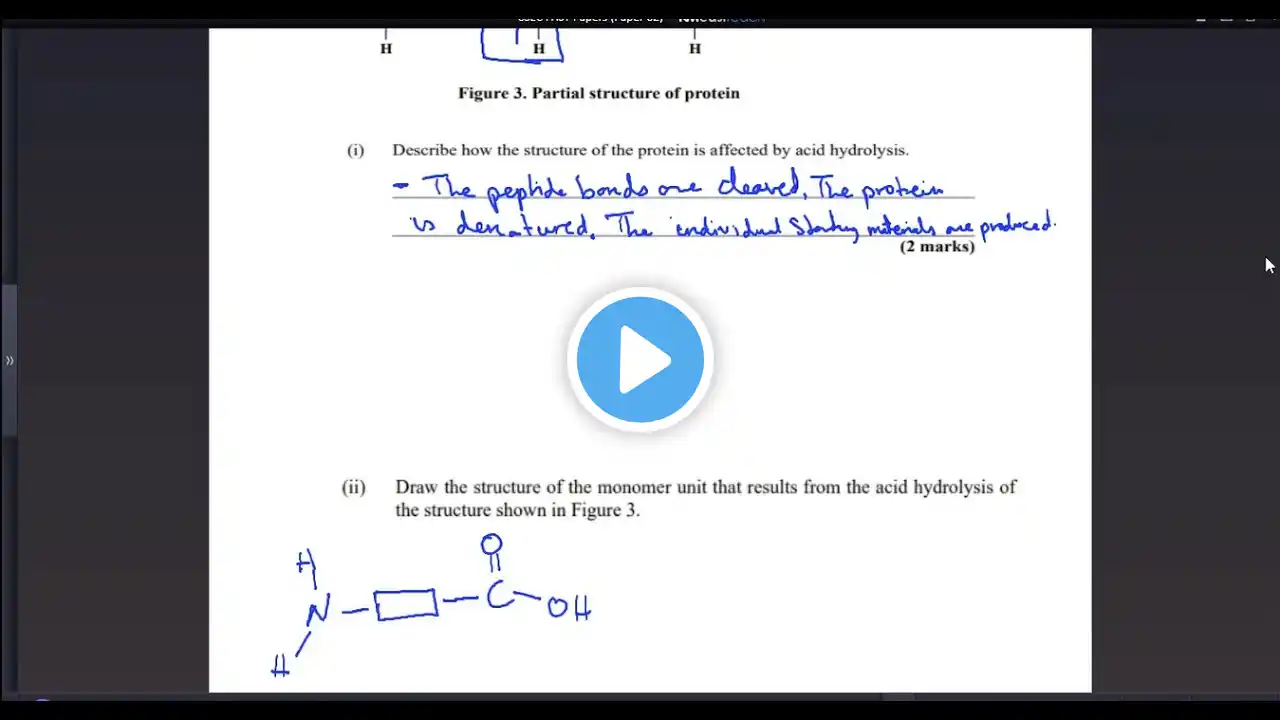
CXC CSEC Chemistry ~ June 2013 Paper 1 ~ Q&A Timestamps: 01 ~ mass number ~ Q & A 0:10 02 ~ electrons, protons, neutrons ~ Q & A 0:25 03 ~ oxide of a metal ~ Q & A 0:40 04 ~ supplies protons ~ Q & A 0:55 05 ~ neutralization ~ Q & A 1:10 06 ~ group number of an element ~ Q & A 1:25 07 ~ arrangement of element on the periodic table ~ Q & A 1:40 08 ~ equal quantities of atoms ~ Q & A 1:55 09 ~ heated lead nitrate equation ~ Q & A 2:10 10 ~ hydrogen bromide ~ Q & A 2:25 11 ~ ionic compound with sulfur and something like sodium ~ Q & A 2:40 12 ~ giant molecular structure ~ Q & A 2:55 13 ~ allotropy ~ Q & A 3:10 14 ~ ionic compounds ~ Q & A 3:20 15 ~ covalent compounds ~ Q & A 3:35 16 ~ sedimentation ~ Q & A 3:50 17 ~ separating funnel ~ Q & A 4:05 18 ~ hydrogen and group 7 ~ Q & A 4:20 19 ~ pH of fresh cane juice ~ Q & A 4:35 20 ~ salt prepared by precipitation ~ Q & A 4:50 21 ~ not form a normal salt ~ Q & A 5:05 22 ~ weak acid ~ Q & A 5:20 23 ~ reduced compound ~ Q & A 5:35 24 ~ soluble in water ~ Q & A 5:50 25 ~ weak electrolyte ~ Q & A 6:05 26 ~ metal atom becomes ion ~ Q & A 6:20 27 ~ sulfur dioxide and oxidizing ~ Q & A 6:33 28 ~ bulb brightness ~ Q & A 6:45 29 ~ does not conduct electricity ~ Q & A 7:00 30 ~ graphite electrodes ~ Q & A 7:15 31 ~ catalyst ~ Q & A 7:29 32 ~ magnesium and sulfuric acid reaction curve ~ Q & A 7:44 33 ~ exothermic reaction ~ Q & A 8:00 34 ~ Haber process ~ Q & A 8:14 35 ~ conducts electric current and remains chemically unchanged ~ Q & A 8:28 36 ~ which nitrate ~ Q & A 8:42 37 ~ physical properties and a metal ~ Q & A 8:56 38 ~ non-metal ~ Q & A 9:10 39 ~ extracted by chemical reduction ~ Q & A 9:25 40 ~ does not form acidic oxide ~ Q & A 9:39 41 ~ potassium chloride and silver nitrate ~ Q & A 9:54 42 ~ alkaline gas ~ Q & A 10:10 43 ~ producing ammonia ~ Q & A 10:23 44 ~ potassium iodide and yellow precipitate ~ Q & A 10:37 45 ~ electropositive ~ Q & A 10:51 46 ~ homologous series ~ Q & A 11:05 47 ~ homologous series structure ~ Q & A 11:22 48 ~ structure of a compound ~ Q & A 11:35 49 ~ name that compound ~ Q & A 11:50 50 ~ esters ~ Q & A 12:05 51 ~ propene and bromine ~ Q & A 12:20 52 ~ nickel ~ Q & A 12:35 53 ~ alcohol and a carboxylic acid ~ Q & A 12:45 54 ~ proteins formed from amino acids ~ Q & A 13:03 55 ~ describe the reaction ~ Q & A 13:18 56 ~ cracked large alkane molecules ~ Q & A 13:33 57 ~ major natural source of alkanes and alkenes ~ Q & A 13:48 58 ~ fermentation of sugars ~ Q & A 14:03 59 ~ hydrolyzed protein ~ Q & A 14:18 60 ~ polymer structure and monomer ~ Q & A 14:33 May/June P1-Solutions CSEC Chemistry Playlist • CXC/CSEC Chemistry June Paper1s January P1-Solutions CSEC Chemistry Playlist • CXC/CSEC Chemistry January Paper1s All P1-Solutions CSEC Chemistry Playlist • CXC/CSEC Chemistry Paper 1s P2-Solutions: • Solutions ~ CSEC Chemistry P2s