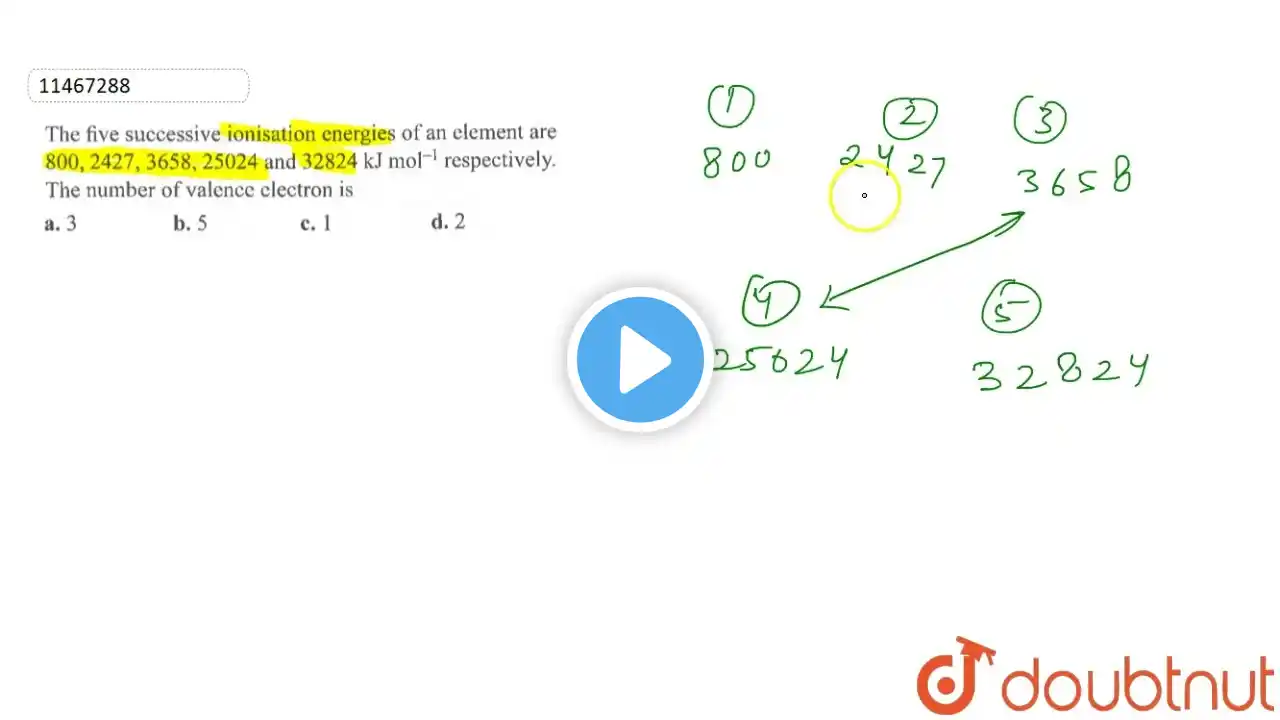
The successive 5 ionization energies of an element are 800, 2427, 3658, 25024 and 32824 kJ/mol,
The successive 5 ionization energies of an element are 800, 2427, 3658, 25024 and 32824 kJ/mol, respectively. By using the above values predict the group in which the above element is present: (1) Group 2 (2) Group 13 (3) Group 4 (4) Group 14 #11thchemistry #jeechemistry #jeemains #jee2025