How do Acids React with Metals ! अम्ल की धातु से क्रिया #Physics #razisir #onestepforeducation
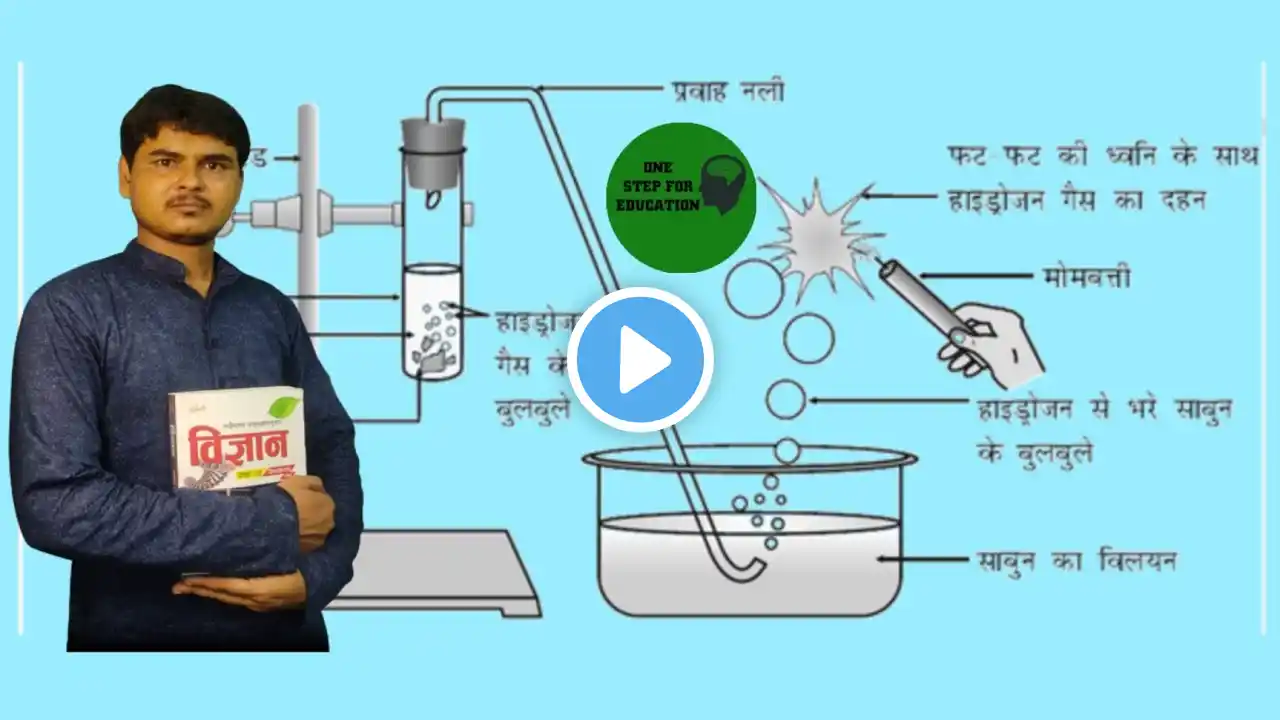
#razisir #razi_sir #razi sir #razi_sir_Mr #onestepforeducation #one_step_for_education #one step for education #amuic #ameerul #रज़ीसर #रज़ी_सर #अमीरूल #mathmasti #math_masti #upboard2023 #exam2023 #biharboard2023 #upboardexam2023 #onestep for education #byrazisir #Class10 #ncert #ncertsolutions #cbse #cbseboard #cbseclass10 #upboard #what #hcl #hclrecruitment #acid #Reaction of Acids with Metals #अम्ल की धातु से क्रिया #Physics acid bases and salts acid react with metal acids bases and salts activity 2.6 class 10 chemistry activity 2.6 class 10 science activity 2.6 class 10 science answers activity 2.6 class 10 science observation activity 2.6 class 10 science solution chapter 2 class 10 class 10 science class 10 science chapter 2 class 10 science chapter 2 activity 2.6 learn practically mayank sir what happens when acid reacts with metal Iron filings, Fe(s) – see CLEAPSS Hazcard HC055a. Magnesium ribbon, Mg(s) – see CLEAPSS Hazcard HC059a. Magnesium turnings are HIGHLY FLAMMABLE. Distribution of pieces of magnesium ribbon should be supervised to avoid students taking several pieces and experimenting later with igniting them. Zinc granules, Zn(s) – see CLEAPSS Hazcard HC107. While other metal/acid combinations react in the same way, recovering the salt by crystallisation (in Lesson 2) may not be as successful as it is using zinc and sulfuric acid. Dilute hydrochloric acid, HCl(aq) – see CLEAPSS Hazcard HC047a and CLEAPSS Recipe Book RB043. Dilute sulfuric acid, H2SO4(aq), (IRRITANT at concentration used) – see CLEAPSS Hazcard HC098a and CLEAPSS Recipe Book RB098. Procedure Lesson 1 Place six test tubes in the test tube rack. Add a 2–3 cm depth of dilute hydrochloric acid to the first three tubes, and a 2–3 cm depth of dilute sulfuric acid to the remaining three tubes. Add a small piece of a different metal to each of the tubes with hydrochloric acid in them. Record which metal you add to each tube. How do Acids and Bases React with Metals - YouTube h://www EVIEW 6:57 In this Chemistry video for Class 10 in Hindi we explained how acids and bases react with metals. Add a small piece of the same metals to each of the tubes with sulfuric acid in them. Record which metal you add to each tube. Your teacher will show you how to test the gas being produced in these reactions. Related searches reaction of acids with metals experiment sulphuric acid and metal reaction reaction of metals with acids reaction of acids and bases with metals lab activity reaction of metals with dilute hydrochloric acid acid reaction how do metal carbonates and metal hydrogencarbonates react with acids what happens when hydrochloric acid reacts with sulphuric acid Q.3: धातु के साथ अम्ल की अभिक्रिया होने पर सामान्यत: कौन-सी गैस निकलती है? एक उदाहरण के द्वारा समझाइए। इस गैस की उपस्थिति की जांच आप कैसे करेंगे? उत्तर : जब धातु के साथ अम्ल अभिक्रिया करते हैं तब प्राय: हाइड्रोजन गैस उत्पन्न होती है। Zn (s) + H2SO4 (dil) → ZnSO4 (aq) + H2 (g) जिंक सल्फ्यूरिक अम्ल जिंक सल्फेट हाइड्रोजन हाइड्रोजन गैस को साबुन के घोल से गुजारो। बुलबुले उत्पन्न होंगे। उन बुलबुलों के निकट जलती हुई मोमबत्ती की ज्वाला लाओ। वे फट-फट की ध्वनि के साथ जलेंगे। इस से हाइड्रोजन गैस की उपस्थिति सिद्ध हो जाती है।