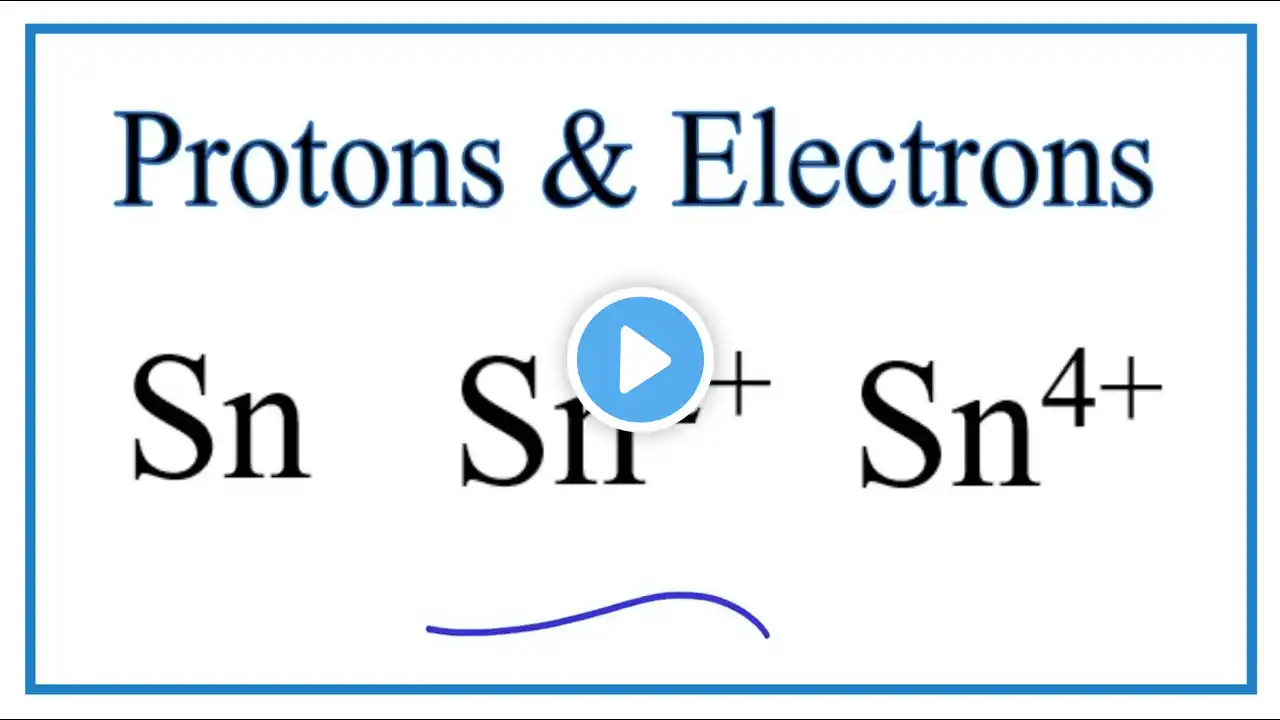
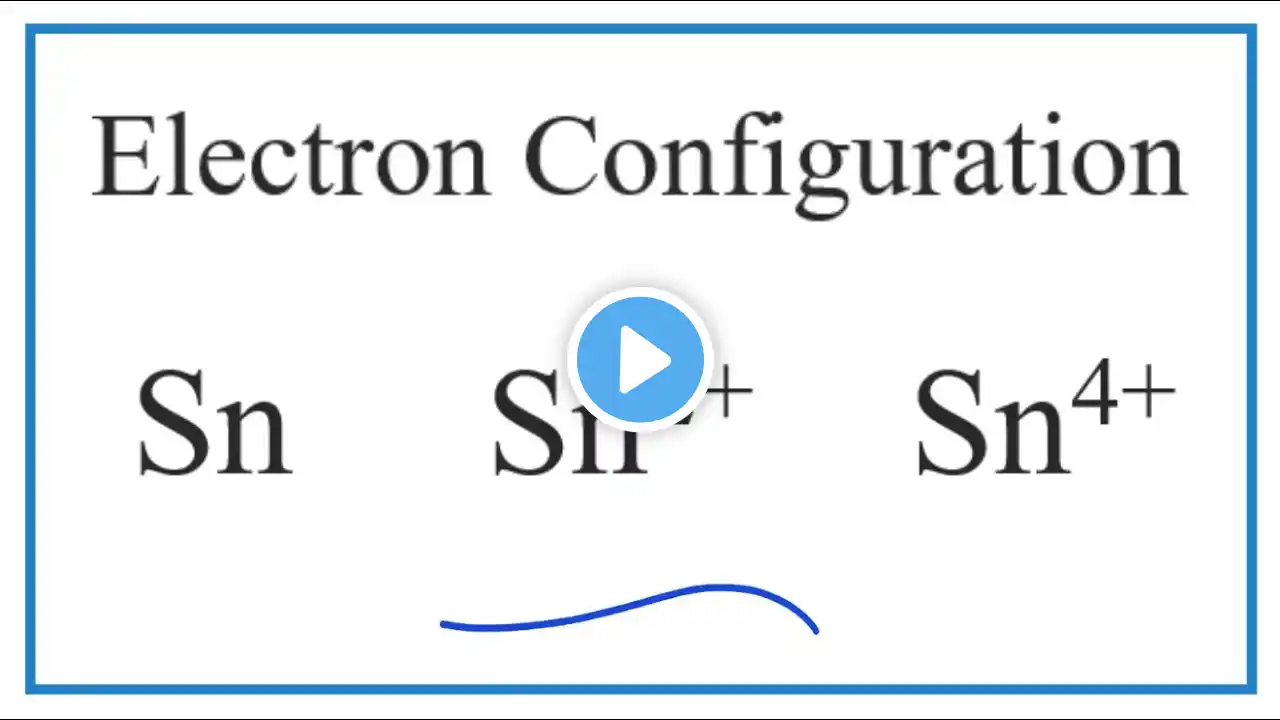
How to Find the Valence Electrons for Tin (Sn)
To find the number of valence electrons for Tin (Sn) we need to look at its electron configuration. This is necessary because Sn is a transition metal (d block element) and we need to take into account electrons found in its d orbitals. We can write this in condensed electron configuration for Tin as: [Kr] 4d¹⁰5s²5p² How to write the Sn electron config: • Electron Configuration for Sn, Sn 2+,... This allows us to look at the number of electrons outside of the Noble Gas core. For Sn this means we have 14 valence electrons. However since Tin normally forms 2+ and 4+ ions it could be argued that the 5s²5p² represent the valance electrons for Sn. Note that for transition metals, like Tin, not all of the valence electrons have to be used to form chemical bonds with other elements. More on Valence Electrons for Transition Metals: https://en.wikipedia.org/wiki/Valence... Definition of Valence in Transition Metals: Miessler G.L. and Tarr, D.A., Inorganic Chemistry (2nd edn. Prentice-Hall 1999). p.48. Helpful videos: • Finding Valence Electrons for Transition Metals: • How to Find the Number of Valence Ele... • Finding Valence Electrons (element): • Finding the Number of Valence Electro... • Finding Valence Electrons (molecule): • Finding the Number of Valence Electro... • How to Draw Lewis Structures: • How to Draw Lewis Structures: Five Ea... Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).